Treatments
Multidisciplinary Care
Multidisciplinary care is a collaborative approach to treatment planning and ongoing care. It is well documented and accepted that multidisciplinary care represents ‘best practice’ in terms of treatment planning and care for cancer patients. Effective multidisciplinary care assures:
- Improved treatment
- Improved team communication and support
- Improved coordination of patient care
- Reduced service duplication
- Increased opportunities for recruitment into clinical trials
- Consideration of patient’s physical and emotional needs

-
Breast Tumor Board
Physicians within our program, collaborate weekly, in our Breast Tumor Board. At this meeting, all specialties are represented, and, cases are presented, x-rays and pathology slides reviewed, and, discussion takes place, regarding the most appropriate treatments for each case. This method essentially allows for multiple ‘opinions’ from each specialty.
Immediately following Tumor Board, patients, their families, and the rendering physicians, meet independently, to discuss the recommendations from Tumor Board. Patients have an opportunity, at this meeting, to have all of their providing physicians, in one room, available for discussion regarding any particular questions/concerns. In addition, other cancer ‘services’ provided via our Cancer Center, are presented, offering a global overview of the care plan.
The care provided for breast cancer patients, include multiple specialists: pathology, radiology, medical oncology, and radiation oncology. Other support services are equally important: social workers, genetic counselors, physical therapists, clinical trials, nutritional experts, members of our exercise and wellness program, and survivorship personnel.
Neoadjuvant Therapy
Neoadjuvant therapy is systemic therapy given BEFORE surgery. There are two types of neoadjuvant therapy: chemotherapy and endocrine (anti-estrogen) therapy. The purpose of preoperative therapy is to potentially downstage the tumor, optimize surgical outcomes, and reduce the risk of breast cancer recurrence.
-
Who Is Appropriate for Neoadjuvant Therapy?
Neoadjuvant therapy is typically used in the face of high-risk breast cancers. High-risk breast cancers are those with a high rate of distant spread and mortality without the use of modern local and systemic therapies. Neoadjuvant systemic therapy may be considered for any patient for whom adjuvant systemic therapy is indicated. The use of neoadjuvant therapy is a multidisciplinary decision – specific goals should be outlined.
- Locally Advanced Breast Cancer
- Inflammatory Breast Cancer
- Downstaging Large Tumors to allow for breast conservation (lumpectomy)
- Need to ‘delay’ surgery for medical reasons
-
Potential long-term benefits
- Assess the effectiveness of therapy
- Downsize tumor in the breast
- Downstage Axilla
- Lumpectomy instead of Mastectomy
- Allow for genetic testing results that may affect surgical decision
- Determine who may require additional systemic therapy:
a. Triple Negative Cancer: Capecitabein/Xeloda
b. HER2 positive Cancer: Ado-Trastuzumab/Kadcyla
-
Potential candidates for neoadjuvant therapy
- Larger tumors in a smaller breast
- Node positive at presentation
- HER2 postive tumors
- ‘Triple Negative’ tumors
-
Who is inappropriate for neoadjuvant therapy?
- Patients who present without a definitive indication for chemotherapy
- Patients not motivated to preserve their breast and/or desire mastectomy
-
The Latest Trials
Study of 1,486 patients with HER2 positive cancers who had residual disease found on surgical pathology. Those with residual disease were randomized to traditional treatment or T-DM1 (trastuzumab emtansine). Risk of recurrence or death was 50% lower in T-DM1 group
Breast Surgery
The are many surgical treatments for breast disease, ranging from open surgical biopsies, to lumpectomy, mastectomy, and, sentinel lymph node biopsy. The appropriate preoperative evaluation is paramount to making the correct surgical choice.
-
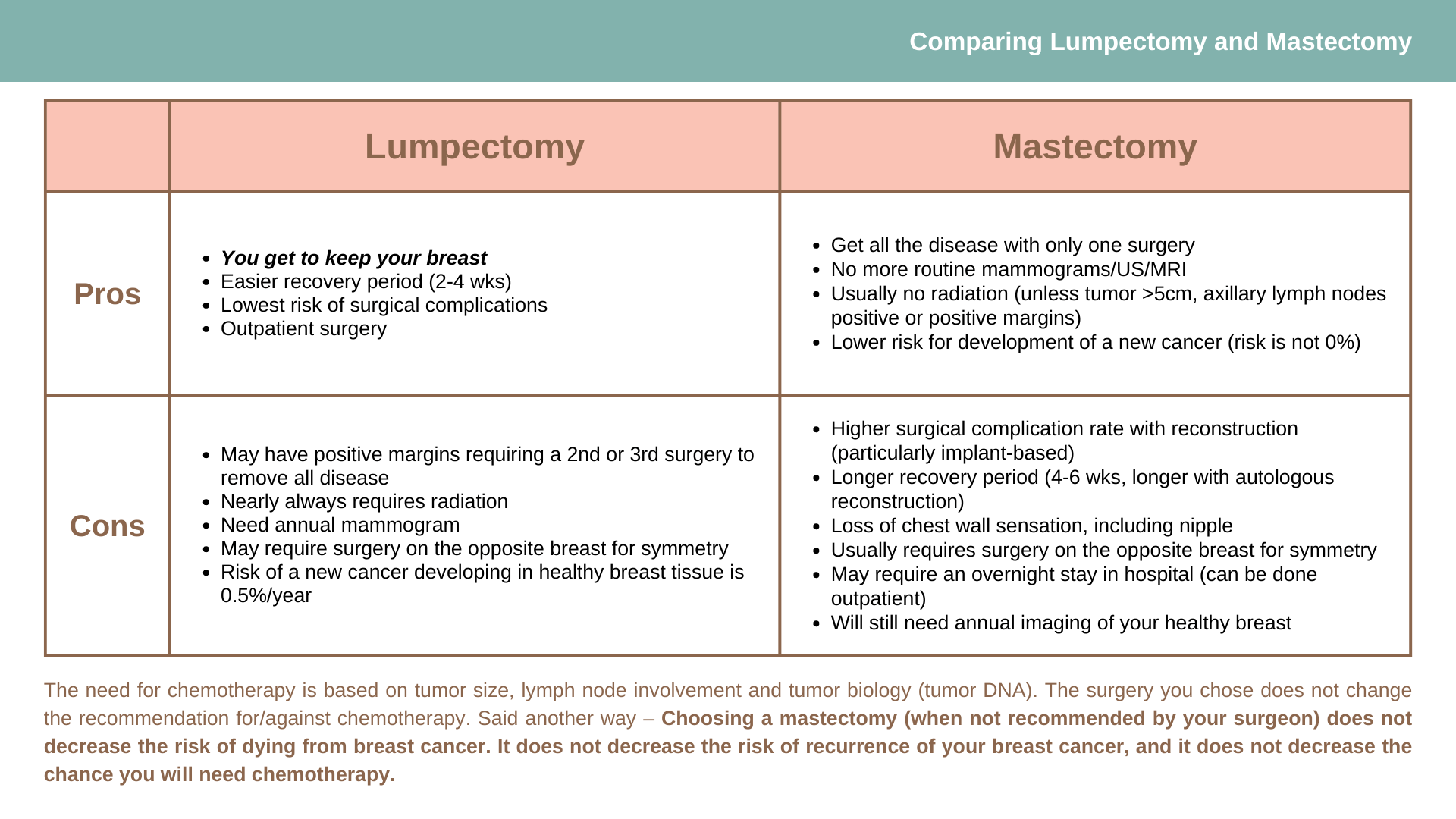
Lumpectomy
Lumpectomy, or breast conservation, typically includes adjuvant radiation therapy and has been found equivalent to mastectomy in terms of long-term survival. The procedure involves the removal of cancerous tissue as well as a rim of healthy tissue, called a margin. The need for a negative/clear margin is critical. Adjuvant radiation therapy is necessary in most cases where lumpectomy is the chosen surgical treatment. Radiation is given to the breast to address any potential microscopic disease is ‘left behind’. Local recurrence rates (the chance of the same cancer reoccurring in the breast) with lumpectomy AND radiation therapy is approximately 2-3%; whereas the recurrence rate without radiation therapy is approximately 30%.
Utilizing advanced surgical techniques, called Oncoplastic techniques, the defect created by a partial mastectomy can be repaired. Following lumpectomy and radiation, the appearance of the breast should be relatively the same as preoperatively.
-
Mastectomy
Mastectomy involves removal of all of the breast tissue. In reality however, there is a small amount of microscopic breast tissue that remains. Thus, the local recurrence rate (the chance of the same cancer reoccurring in the breast) is approximately 2-3%. This is as low a local recurrence rate that can be achieved. Most importantly however, the overall survival is no different than a lumpectomy.
There are different types of mastectomies: skin sparing mastectomies remove all the breast tissue, including the nipple and areola, leaving behind most, if not all of the native skin. These procedures are typically done in conjunction with reconstruction. Nipple/areolar reconstruction can be performed by a plastic surgeon, at a later date; nipple sparing mastectomies remove all of the breast tissue; the nipple/areolar complex is not removed and remains in place; areolar sparing mastectomies remove all of the breast tissue, including the nipple; the areola remains. The nipple is reconstructed by a plastic surgeon, at a later date.
The indications, risks, and benefits of each mastectomy option differs slightly, as does the indication to consider each.
-
Contralateral Prophylactic Mastectomy
Understandably, when diagnosed with breast cancer, many women are of the opinion: "Just remove both my breasts so I won't have to worry anymore". Despite what may seem so obvious, the data simply do not support this assertion.
The performance of mastectomy in general has recently undergone significant scrutiny. There is simply no survival advantage offered by mastectomy over breast conservation. Therefore, removing a contralateral unaffected breast will have no clinical benefit.
The American Society of Breast Surgeons came out with a consensus statement regarding CPM in June, 2016:
CPM should be considered for those at significant risk of CBC (contralateral breast cancer)
- Documented BRCA1/2 carrier.
- Strong family history, but patient has not undergone genetic testing.
- History of mantle chest radiation before age 30 years can be considered for those at lower risk of CBC
- Gene carrier of non-BRCA gene (e.g., CHEK-2, PALB2, p53, CDH1).
- Strong family history, patient BRCA negative, no known BRCA family member.
CPM may be considered for other reasons:
- To limit contralateral breast surveillance (dense breasts, failed surveillance, recall fatigue).
- To improve reconstructed breast symmetry.
- To manage risk aversion.
- To manage extreme anxiety. (This may be better managed through psychological support strategies.)
CPM should be discouraged:
- Average-risk woman with unilateral breast cancer.
- Women with advanced index cancer (e.g., inflammatory breast cancer, T4 or N3 disease, stage IV disease).
- Women at high risk for surgical complications (e.g., patients with comorbidities: obesity, smoker, diabetes).
- Woman tested BRCA negative with a family of BRCA-positive carriers.
- Male breast cancer, including BRCA cancer
- CPM
So, what are the facts?
In the ‘average’ patient, the risk of developing a second breast cancer is quite low, and related to patient age range between 25-30% in a 45 year old, depending on biomarkers
CPM does not change the indications for adjuvant (additional) treatments such as chemotherapy, radiation or hormonal (endocrine) therapy.
If a contralateral breast cancer develops it tends to be less relevant (compared to the original cancer)
- more likely to be screen detected (smaller)
- less likely to have metastasized to lymph nodes
- likely to have an excellent prognosis
- The surgical risks of a contralateral mastectomy is higher than unilateral
- Patient education regarding the potential risks and eventual benefits of prophylactic mastectomy is critical
- However: a recent study from Mayo Clinic indicated an 83% patient satisfaction in women undergoing CPM & 84% would choose this option again
-
Reconstruction
Immediate plastic surgical reconstruction, under most circumstances, can be performed at the same setting, if mastectomy chosen as the surgical approach. There are times, however, when a delayed reconstruction might be preferred or recommended. There are a number of different types of breast reconstruction, including placement of an implant, tissue transfer (TRAM Flap, etc) and free flaps (DIEP Flap, etc.). Reconstruction is typically initiated during the initial surgical intervention, but, can also be performed at a later date.
-
Sentinel Lymph Node Biopsy Surgery
In the past, to ‘stage’ breast cancer, a complete axillary lymph node dissection was performed. During this procedure, approximately 15-20 lymph nodes were removed, and, assess histologically (under a microscope). This allowed doctors to assess whether or not cancer cells had begun to spread. In women with clinically negative lymph nodes (those that were not abnormal to physical exam or x-rays), where less likely to have disease in the lymph nodes, so, ultimately negative or clear lymph nodes were removed, but provided no benefit to the patient, only potential side effects. Thus, it was determined that a better mechanism must be found to evaluate lymph nodes for spread of disease.
The sentinel lymph node is the first lymph node(s) that would receive spread of disease, IF, the disease had begun the process of spreading. This lymph node was first utilized in patients who had skin cancer (melanoma); because in these patients the route that cancer spreads is very unpredictable.
To identify this particular lymph node (at times there may be more than one), the breast is injected with a radiotracer and/or a blue dye. These tracers are absorbed by the breast tissue, and, enter the lymphatic system of the breast, ultimately finding their way to the sentinel lymph node. A special probe is utilized intraoperatively, to identify which lymph nodes have the tracer, and, thus the sentinel lymph node(s). By analyzing these nodes, physicians can determine if cancer has begun the process of spreading.
The dye/tracer will drain to the lymph nodes, but, this does NOT mean that the cancer has spread. It is only by analyzing these nodes histologically, that this can be determined.
Research has validated the use of sentinel lymph node biopsy in staging breast cancer. In a trial involving 5,611 women with breast cancer and no clinical signs of axillary metastasis, researchers from the National Surgical Adjuvant Breast and Bowel Project (NSABP), which is a National Cancer Institute (NCI) clinical trials cooperative group, randomly assigned participants to receive SLNB alone or SLNB plus an axillary lymph node dissection (ALND).
The researchers found no differences in overall and disease free survival between the two groups of women. Based on these results, it was concluded that ALND might not be necessary for women with clinically negative axillary lymph nodes and a negative SLNB whose breast cancer is treated with surgery, adjuvant systemic therapy, and external-beam radiation therapy.
-
Surgical Margins
The surgical margin is the distance seen histologically (under the microscope) between the edge of the surgical specimen, and, the cancer within the specimen. This distance is measured, typically in millimeters. When the surgical specimen is removed, it should be oriented by the surgeon, so as to indicate which side of the specimen, corresponds to which location with the surgical cavity in the patient. Typically, there are 6 margins reported. If a margin is too close, it may require re-excision. Re-excision is a second operation, to remove more tissue from an area where the tumor cells were too close, or involving, the edge of the specimen.
Intraoperatively, the margins can be assessed clinically (by touch), radiographically (by performing an x-ray/mammogram/ultrasound of the specimen, to assess how close disease seemed to be to the edge. Recently, a new device, called the Margin Probe, has been used to assess the edges/margins of the surgical specimen. This device uses Radio Frequency (RF) Spectroscopy to determine cellular differences between normal and cancerous tissue. Studies have shown that this device may reduce re-excision rates by as much as 50%. http://www.marginprobe.com/dr-eric-brown/
So, what is the significance of a positive margin? Positive margins (tumor cells seen directly on the edge of the surgical specimen) are associated with a two-fold increase in the risk of local recurrence (IBTR – Ipsilateral Breast Tumor Recurrence) compared with negative margins. There is now a universal consensus (Society of Surgical Oncology and the American Society for Radiation Oncology) that ‘no tumor on ink’ – no tumor to the edge of the surgical specimen, is associated with low rates of IBTR and has the potential to decrease re-excision rates, improve cosmetic outcomes, and, decrease health care costs.
Clinical Trials
Clinical trials are an extremely important component of cancer care. According to National Comprehensive Cancer Network Guidelines, "the best management of any cancer patient is in a clinical trial. Participation in clinical trials is especially encouraged." Drs. Brown, Gold and Richardson actively pursue this mission. Dr. Brown and Dr. Gold served As the PI (Principal Investigator) at Beaumont Health of many of the clinical trials offered by cooperative groups in the U.S. (NSABP, SWOG, Alliance). All three physicians also enroll patients in Clinical Trials through Karmanos Cancer Institute, A National Cancer Institute Designated Cancer Center (one of two in Michigan). Multiple clinical trials are available through the NCI (National Cancer Institute).
-
Chemoprevention
Chemoprevention trials in the US and Europe have evaluated selective estrogen receptor modulators to prevent breast cancer in high risk women. The Breast Cancer Prevention Trial demonstrated that Tamoxifen produced a 49% reduction in invasive breast cancer in US women at increased risk. The Multiple Outcomes Raloxifene Evaluation trial for osteoporosis and the Raloxifene Use of the Heart trial both demonstrated a substantial reduction in the risk of breast cancer in post-menopausal women. Both medications are considered effective as risk reducing strategies in high-risk women. As with most effective treatments, there are side effects that need to be considered (eg. risk of endometrial cancer, stroke, heart disease, cataracts, and bone loss). Proper counseling is essential to this important decision, in the face of high risk.
-
Cooperative Group Clinical Trials
In addition to the Clinical Trials offered through the Karmanos Cancer Institute, here is a list of Beaumont Health Breast Cancer-related Clinical Trials available, as of January 2020.
- ALLIANCE A011202: A RANDOMIZED PHASE III TRIAL EVALUATING THE ROLE OF AXILLARY LYMPH NODE DISSECTION IN BREAST CANCER PATIENTS (CT1-3 N1) WHO HAVE POSITIVE SENTINEL LYMPH NODE DISEASE AFTER NEOADJUVANT CHEMOTHERAPY).
- ALLIANCE A011401 RANDOMIZED PHASE III TRIAL EVALUATING THE ROLE OF WEIGHT LOSS IN ADJUVANT TREATMENT OF OVERWEIGHT AND OBESE WOMEN WITH EARLY BREAST CANCER.
- ALLIANCE A011502 RANDOMIZED PHASE III DOUBLE-BLINDED PLACEBO-CONTROLLED TRIAL OF ASPIRIN AS ADJUVANT THERAPY FOR HER 2 NEGATIVE BREAST CANCER: THE ABC TRIAL.
- ECOG-ACRIN EAY131(MATCH): MOLECULAR ANALYSIS FOR THERAPY CHOICE (MATCH).
- MA.39 TAILOR RT: A RANDOMIZED TRIAL OF REGIONAL RADIOTHERAPY IN BIOMARKER LOW-RISK NODE-POSITIVE BREAST CANCER.
- NRG BR004: A RANDOMIZED, DOUBLE-BLIND, PHASE III TRIAL OF PACLITAXEL/TRASTUZUMAB/PERTUZUMAB WITH ATEZOLIZUMAB OR PLACEBO IN FIRST-LINE HER2-POSITIVE METASTATIC BREAST CANCER.
- NSABP B-51: A RANDOMIZED PHASE III CLINICAL TRIAL EVALUATING POST-MASTECTOMY CHESTWALL AND REGIONAL NODAL XRT AND POST-LUMPECTOMY REGIONAL NODAL XRT IN PATIENTS WITH POSITIVE AXILLARY NODES BEFORE NEOADJUVANT CHEMOTHERAPY WHO CONVERT TO PATHOLOGICALLY NEGATIVE AXILLARY NODES AFTER NEOADJUVANT CHEMOTHERAPY.
- SWOG S1418/BR006: A RANDOMIZED PHASE III TRIAL TO EVALUATE THE EFFICACY AND SAFETY OF MK-3475 AS ADJUVANT THERAPY FOR TRIPLE RECEPTOR-NEGATIVE BREAST CANCER WITH GREATER THAN 1CM RESIDUAL INVASIVE CANCER OR POSITIVE LYMPH NODES (>PN1MIC) AFTER NEOADJUVANT CHEMOTHERAPY.
- SWOG S1706: TO COMPARE THE INVASIVE DISEASE-FREE SURVIVAL (IDFS) OF PATIENTS WITH INFLAMMATORY BREAST CANCER RECEIVING CONCURRENT ADMINISTRATION OF OLAPARIB WITH STANDARD DOSES OF RADIOTHERAPY TO THE CHEST WALL AND REGIONAL LYMPH NODES COMPARED TO STANDARD DOSES OF RADIOTHERAPY ALONE TO THE CHEST WALL AND REGIONAL LYMPH NODES.
As members of the Karmanos Cancer Institutes Breast Multidisciplinary Team and Beaumont Health Cancer Centers, the physicians of Comprehensive Breast Care have access to multiple NCI-supported clinical trials.
-
Industry Sponsored Clinical Trials
Comprehensive breast care is committed to providing our patients with access to as many clinical trials as possible. The trials listed here are sponsored by industry or commercial companies. These trials are also important in continuing the quest for the best and most appropriate options for our patients. Many of these trials will be "practice changing" with a tremendous impact on patient care.
TME Universal Gene Study: iGap
Project Goal:
Understand the incidence of deleterious mutations in the general breast cancer patient population and whether the criteria by which patients are currently selected for testing are adequate. A total of 1000 patients will be accrued across 15 to 20 breast surgery sites and tested with Invitae’s 80-gene multi-cancer panel (participating clinicians can also select to receive STAT results upfront for up to 9 genes to aid in surgical decisions). Target accrual for each site is 50 patients (25 in-criteria and 25 out-of-criteria patients).
Data to be collected includes:
- Patient demographics and diagnosis, test results, and neo-adjuvant treatment plan;
- Immediate post-treatment plan and results, including what treatment/s employed and pathologic results;
- Patient interim follow up including status of breast cancer and patient at a 1 and then 3 year time period
Curebase B4 Trial
The B4 study is looking at how effective an investigational breast cancer screening test using blood only compares to the traditional methods of mammograms and breast biopsies. The B4 Study is enrolling participants that are scheduled for a screening mammogram or who are getting a breast biopsy following a screening mammogram. The participants will be required to complete one blood draw and the study will collect the results of the screening or diagnostic procedures already being completed as part of your normal standard of care. Your participation has a BIG impact. Ask our doctors about the B4 study today.
The B4 study is being conducted at:
Comprehensive Breast Care
4967 Crooks Rd.
Suite 210
Troy, MI 48098
If you want to learn more about participating in the B4 study, please contact us at (248) 687-7300 or visit https://www.compbreastcare.com/contact-us
Agendia Flex Trial
FLEX is a large-scale observational study of recently diagnosed breast cancer patients, aimed at discovering more about how breast cancer differs from patient to patient. Women and men who have been diagnosed with stage I, II, and III invasive breast cancer are eligible. Your participation has a BIG impact. Ask our doctors about FLEX today.
The FLEX study is being conducted at:
Comprehensive Breast Care
4967 Crooks Rd.
Suite 210
Troy, MI 48098
If you want to learn more about participating in the FLEX study, please contact us at (248) 687-7300 or visit https://www.compbreastcare.com/contact-us
Radiation Therapy
Radiation therapy, or radiotherapy, is used to treat breast cancer by killing cancer cells in an area that has been specifically targeted. After a lumpectomy breast radiation reduced the risk of local recurrence. Radiation effectively ‘cleans up’ any remaining microscopic cancer cells that may have remained after surgery. Essentially, radiation is therapeutic doses of x-ray beams designed to kill cancer cells.
-
Why Is Radiation Therapy Used?
Multiple studies have shown that women who received radiation had a significant reduction of local or distant recurrence when compared to those who did not undergo radiation. They also found a significant reduction in the risk of death by breast cancer in those treated with radiotherapy. Thus, the patients who had radiotherapy when radiotherapy was indicated did better than those who did not receive radiotherapy. This is why radiation is included as part of the therapy for breast cancer. It is intended to kill microscopic disease that cannot be seen or felt, that may be left behind after surgery.
-
When Is Radiation Therapy Used?
Radiation is primarily used in the following settings:
- After partial mastectomy (lumpectomy)
- After mastectomy (whole breast removal), if the cancer invades into the chest wall or through the skin
- After mastectomy and axillary staging if more than 4 nodes were positive. If 1 to 3 nodes were positive after mastectomy, radiotherapy may be recommended
-
How Is Radiation Therapy Administered?
Radiotherapy can be administered in two ways:
- Whole-breast radiation therapy - Whole-breast radiation therapy treats all remaining breast tissue after a partial mastectomy (or lumpectomy). This therapy is delivered in daily doses over 4 to 6 weeks, typically Monday through Friday. In each session the patient lies in a machine that delivers the radiation, which is targeted on the breast tissue.
- Partial-breast radiation therapy - Allows radiation to be administered directly to the lumpectomy cavity, and spares surrounding breast tissue. The radiation is applied directly to the tissue at the surface of the lumpectomy cavity, as well as a 1 cm depth of tissue. Because of the sophisticated planning that APBI allows, vital structures, such as the skin and chest wall, can receive lower dosages. Partial breast irradiation can typically be completed in 5 days; having 2 treatments per day (separated by about 6 hours). APBI is very well tolerated, being associated with almost no side effects. Long term local control results are similar to those achieved with whole breast radiation.
There are lifetime limits to the amount of radiation that can be given to a specific area of the body and this limit will determine the dosage of the radiation therapy. The physician who delivers radiation therapy (the radiation oncologist) will work with the breast surgeon and patient to determine the most suitable treatment. It is very important that you take care of your skin during radiation. Use moisturizer liberally – about 3-4x day. Jean’s Cream is the most effective, but you can also use OTC agents such as Aquaphor. It is very important that you wear a soft, cotton, supportive bra without underwire. MAKEMERRY is a wonderful option – the bra is extremely comfortable and designed by a female radiation oncologist/company owner specifically for women going through breast cancer treatment.


